Solid-state: the adjective to describe the most pivotal moment in battery innovation, if it ever happens, of course.
Most car makers have muttered something about them in the last couple of years, but what are they and why should you care. Here’s all you need to know about this ground-breaking tech, right down to when it'll be available in our EVs.
OTHER STORIES YOU MIGHT HAVE MISSED:
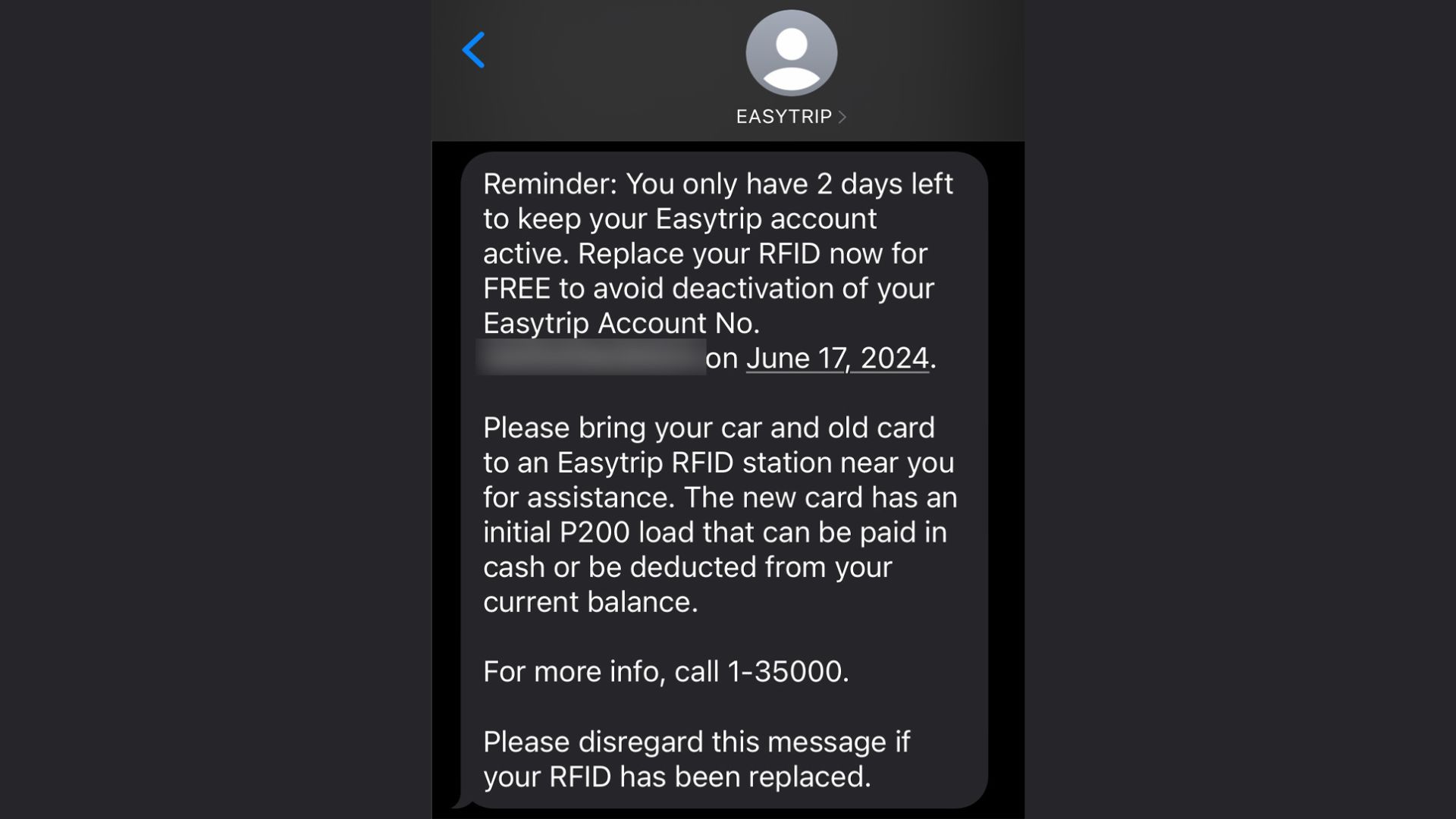
Did anyone receive a seemingly suspicious text message from Easytrip?
What’s the gasoline-electric Nissan X-Trail really like to drive?
What is a solid-state battery?

It’s a battery that uses a solid electrolyte, instead of a liquid or gel-based one. The electrolyte is that bit in the middle, between the cathode and anode.
Why are solid-state batteries the next big thing for EVs?
Solid-state battery compositions will make batteries smaller and more energy dense. That means an EV can either go further with more batteries, or do the same range but be more lightweight and, crucially, cheaper with fewer batteries.
Also, the technology better supports rapid charging, due to its ability to not get so hot. Consider all the carbon-minimal motorsporting potential.
So, how does it all work?
Let's simplify things a minute: picture a 10cm wide rectangle. The first two centimetres are the anode (positive electrode) – usually made from graphite* – and the last two centimetres are the cathode (the negative electrode) – made from lithium iron phosphate (LFP) or lithium nickel manganese cobalt oxide (NMC)*.
(*others are being tested, but we’ll come onto that)
Between the anode and cathode sits a liquid or gel electrolyte. In the middle sits a separator – a type of salt membrane that catalyses the chemistry, encourages the electrons to move between the two electrodes and prevents the electrodes from touching.
Okay, they're definitely not supposed to do that.
You're absolutely right. All rechargeable batteries suffer from a condition called dendrite formation. It’s a bit like dental plaque build up, but for electrodes. The repeated charging and discharging causes ion particles to form long spiky structures on the surface of the electrode. If they get so long they meet, they can cause the battery to short, or worse still catch fire. Hence, the separator.
Right. How's this going to change in a solid-state battery?

By switching the electrolyte to a glass, ceramic or solid polymer one, you can achieve a few helpful things.
Firstly, you can stop liquidy stuff sloshing around in the battery. Glass, of course, does not slosh, and sloshing is not especially stable and cars, by their very nature, tend to move. Unsurprisingly, car makers like stability. Solid electrolytes are more stable (and also less flammable, for the less-fiery-win).
Secondly, the solid electrolyte can multi-task as the catalysing separator, too. Doing away with the separator and the liquid electrolyte means your 10cm rectangle is now only six centimetres wide. A two-centimetre electrolyte sandwiched between a two-centimetre anode and two-centimetre cathode – solid-state sandwich heaven.
That makes it more energy dense than current batteries, right?
Exactly, the energy density of the battery already increases with the reduction in size. But using solid-state electrolytes means a graphite anode could be switched with a lithium one.
A lithium anode could improve the energy density of the cell by as much as 40 %.
And wait, that’s not all. Today's batteries need plenty of thermal management. Slower charging, even in rapid mode, kicks in at 50-80 % of charge to prevent overheating. As such you rarely get full use of all the battery and charging takes longer.
Solid-state batteries are capable of withstanding more heat, so they can be charged more rapidly and there's fuller use of all the cells.
What's more, researchers have found the heat generated from rapid charging increases 'ionic conductivity'. That appears to inhibit the growth of our spiky dendrite foes. Some studies even show certain temperatures ‘self-healing’ dendrites, meaning the structure becomes less spiky and shorter. Pretty wild, eh?
But why aren't solid-state batteries in the market yet?

Great question. Although theoretically, solid-state batteries will be more durable, the folks in lab coats haven’t quite gotten them to that point yet.
That, coupled with the difficulties and costs associated with scaling new tech means it’ll be a little while before solid-state batteries are in our cars. Some niche/premium models might feature them by the back end of the decade.
Plus, the way batteries are currently produced, if scientists want to change the materials of the anode and/or cathode, it’s pretty straightforward. Retooling for switching out the electrolyte, however, isn’t so easy – change involves expense and car makers are trying to drive down the costs, so there's that.
Anything else to know about these batteries?
Solid-state batteries aren’t a new invention at all. Michael Faraday, godfather of many electrochemical and electromagnetic principles and inventor of the aptly titled Faraday Cage, dabbled with the stuff long before our time.
NOTE: This story first appeared on TopGear.com. Minor edits have been made.